Risk In Quality Assurance Department
Potential failure to adhere to specifications. ISO 90002015 is about enrolling everyone in Quality Risk in ISO 90002015 is simply stated but maps well to the risk methodology Figure out your path to risk and leverage tools to expand to a risk-based QMS There are tools to help ease this transition.

Internal Quality Management System Internal Audit Checklist Quality Management System Internal Audit
For developing a risk management RM quality program.

Risk in quality assurance department. Quality defines the value of your products and services and can include a wide range of factors. Quality Assurance extends to all aspects of data collection from sanitary surveys to laboratory procedures. Assurance and enhanced decision-making.
3 Definitions Quality Assurance QA Pertaining to a comprehensive approach or system for ensuring product quality. To subject the therapeutic success to a statistical analysis markers of success are described. Quality Assurance in Software Testing.
RFG believes using outsourced application development services can expose enterprises to. The other topics are the fundamentals of risk management. This chapter deals in detail with the specific aspects of quality management.
Quality assurance QA. Test professionals can avail themselves of three powerful techniques for analyzing risks to system quality. The outcomeresult of the risk assessment process should be appropriately communicated and documented as per Annexure-V.
66326 Risk communication is information sharing session between Quality Risk Management Team and other concerned departments senior management involved with different functions. Social Sciences Humanities Open Cloud hereinafter also referred to as SSHOC project reference. 15 Corporate Management inadequate sponsorship project management.
The quality assurance department must operate independently from the operational units and it must regularly perform quality review activities self-inspection auditsinternal audits to ensure compliance within operational units with Company quality standards good working practices GxPs. A quality control plan simply summarizes the potential errors for a device and how the laboratory intends to address the risk of those errors. In the case of a substandard product being produced it has to be kept away from the reach of.
Current Good Manufacturing Practice cGMP Good Laboratory Practice GLP GCP etc and local. Quality risk is the potential for losses due to quality that fails to meet your quality goals. Quality Assurance in Software Testing is defined as a procedure to ensure the quality of software products or services provided to the customers by an organization.
Quality assurance focuses on improving the software development process and making it efficient and effective as per the quality standards defined for software products. Successful management and leadership of the quality department for an organization involves an integrated approach that includes a communication network that extends both horizontally and vertically within the organization as well as outward to customers and suppliers. Department of Energy Date name US.
Risk Assessment Program Quality Assurance Plan ESERTM-117R1 November 1997 name Sponsor US. Targeting our testing investment by increasing effort for those areas most at risk results in the highest return on investment. CSO Jan 27 2005 700 am PST.
Background and overview to the ISO 90012015 revision. Quality Assurance Quality Management. The top 10 challenges identified below are the primary constraints to achieving effective quality assurance.
The importance of risk in quality management Whitepaper. These audits determine whether the evaluated activities were appropriately. This RP will identify the quality assurance QA process quality control QC process and related risk management auditing methods for a capital asset portfolio program or project management organization.
Before we can build a high-fidelity test system we have to understand what quality means to our customers. Quality Assurance and Quality Control Chapter 8 84 IPCC Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories 8 QUALITY ASSURANCE AND QUALITY CONTROL 81 INTRODUCTION An important goal of IPCC good practice guidance is to support the development of national greenhouse gas inventories that can be readily assessed in terms of quality and completeness. D12 Quality Assurance Risk Assessment Plan.
Quality Assurance for a recreational water monitoring programme will apart from helping to ensure that the results obtained are corr ect increase the confidence of funding bodies and the public. The potential that products and services will not be fit for purpose. The term quality assurance ensures that the product developed meets quality standards set by the organization before it is being launched into the market.
The systematic and independent examination of all trial-related activities and documents. Long established silos separating departments and divisions and for many organizations can represent a significant change in corporate. A quality control plan can be high level as in this example or very detailed depending on the device the laboratory and the clinical application of the test results.
Thousands of businesses operate daily without a quality management system or program in place. Of the potential individual risks to a GIS project can be grouped under the following categories. Department of Energy Environmental Management Quality Assurance Program Manager Date name Environmental Management and Enrichment Facilities Quality Assurance Specialist Date name.
With the aid of external and internal quality assurance an improvement of therapeutic quality can be initiated. The RP expands on TCM2 sections 114 Quality and Quality Management. This deliverable comprises the quality plan for the SSHOC project and the risk monitoring procedures to be used by project partners.
If a business is content with where it is financially is not concerned about future prosperity loss of market share and customers and expects that its product in its current form will be as viable 50 years from now as it is today a quality management system is an unnecessary burden. Mitigating Risk Through Quality Assurance.

Quality Assurance Mind Map Template Ad Affiliate Assurance Quality Mind Template Map Qa As A Servic Mind Map Template Mind Map Quality Assurance

These Are The Key Elements Of Total Quality Management Upon Which You Can Structure Your Approac Business Management Degree Management Skills Management Degree

Quality Assurance Is A Planned And Systematic Means For Assuring Management That The Defined Standards Change Management Quality Assurance Program Management
Quality Risk Management Qrm For Medicinal Products An Introduction Ivt

عبدالإله منعم On Twitter Internal Audit Swot Analysis Training And Development

D1 2 Quality Assurance Risk Assessment Plan Sshopencloud

Iso9001 Quality Management Systems Infographic A Pictorial Overview Of The Standard Showing The Management Infographic Management Business Process Management

Aurobindo Pharma Ltd Multiple Openings In Qa Aqa Ttd Production Packing Ehs Departments Pharma Walks Latest Pharma J Job Job Website Job Information

Know Your Risks An Infographic Guide Innovation Of Risk Risk Management Management Business Management

Isaha Medical Is Under Construction Quality Assurance Process Control Medical

Patient Safety Risk And Quality

Pin On Project Management Pmo Ppm

Risk Management Maturity Model In 2021 Risk Management Risk Analysis Management

Mylan Bangalore Walk In Interview At Hyderabad For Quality Assurance Interview Risk Management Hyderabad
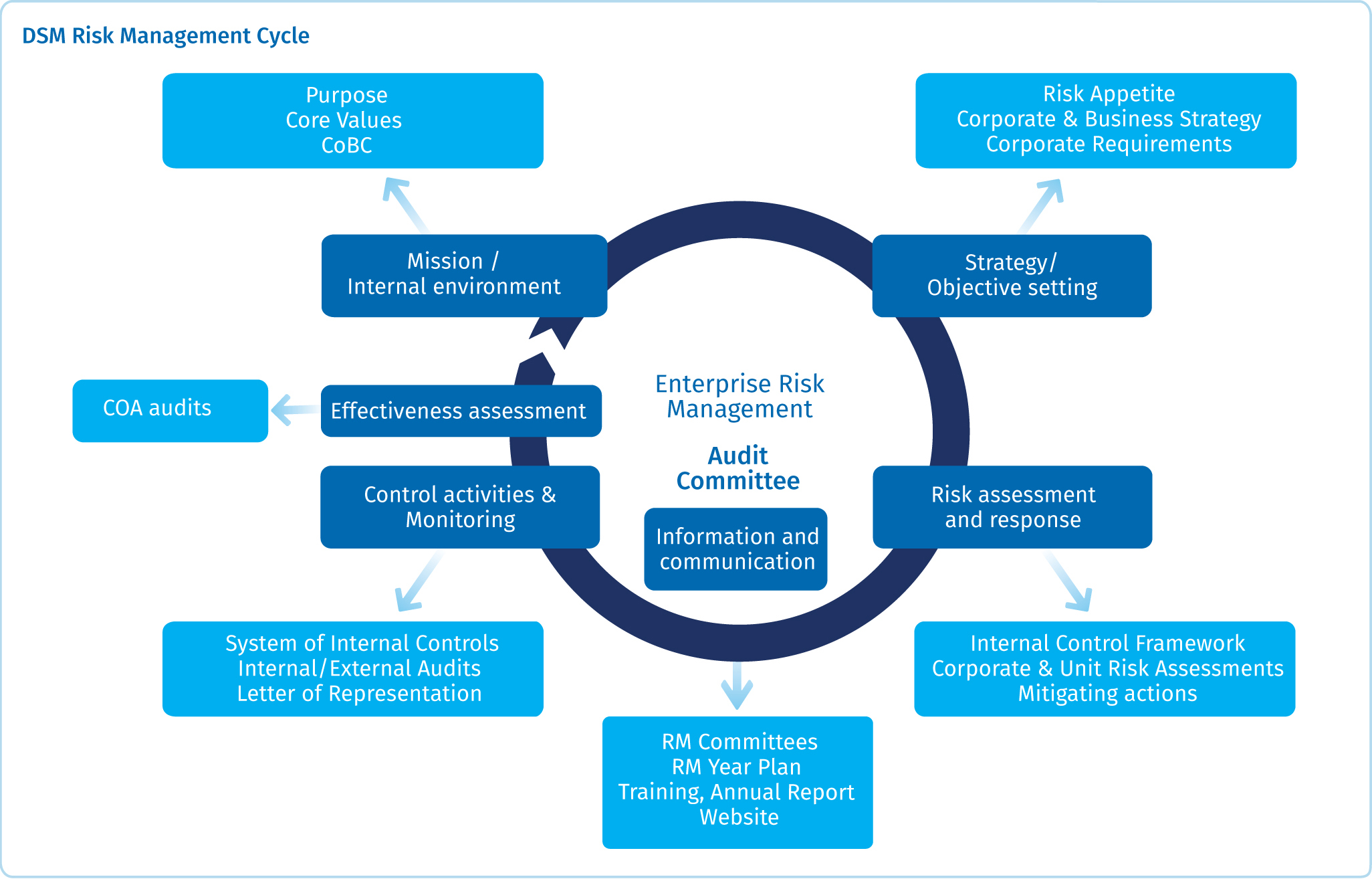
Risk Management Dsm Annual Report

Risk Register Template Download As Excel By Maclaren1 Spreadsheet Quality Assurance Tracking Free Qc Report Business Risk Risk Management Lesson Plan Templates



Posting Komentar untuk "Risk In Quality Assurance Department"